According to the 1998 medical device directive ivdd 98 79 ec an in vitro diagnostic medical device or ivd is defined as any medical device which is a reagent reagent device calibrator control material kit instrument apparatus equipment or system whether used alone or.
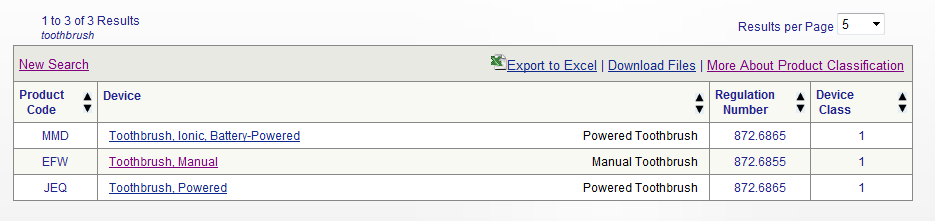
Diagnostic medical devices examples.
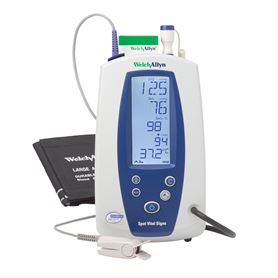
Diagnostic medical equipment and supplies help clinicians to measure and observe various aspects of a patient s health so that they can form a diagnosis.
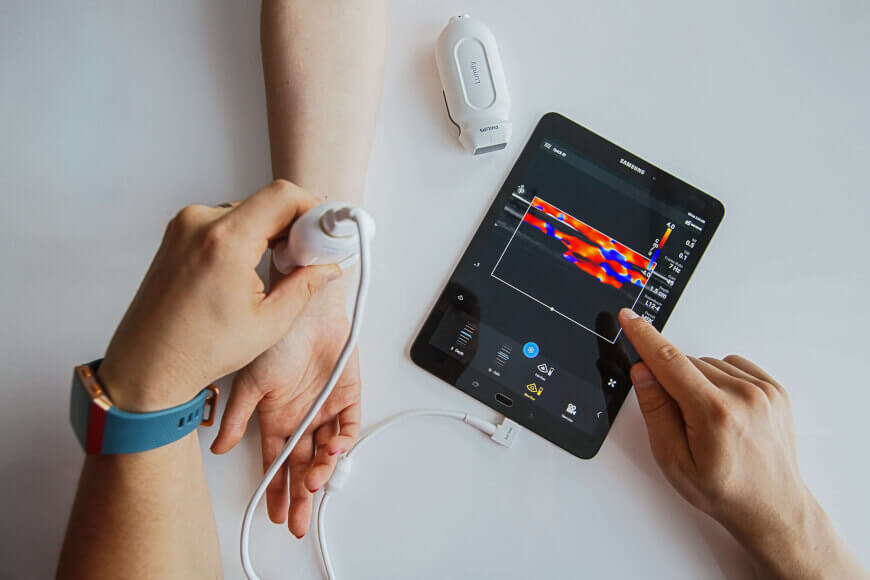
Current health s artificial intelligence ai wearable device that measures multiple vital signs has recently received fda clearance for patients to use at home.
Once a diagnosis is made the clinician can then prescribe an appropriate treatment plan.
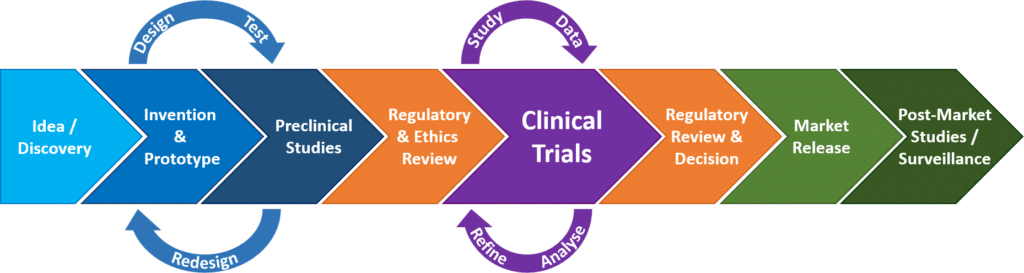
A subgroup of medical products their market access use and market surveillance is regulated.
Medical devices are products or equipment intended generally for a medical use and are regulated at member state level.
The diagnosis treatment mitigation or prevention of a disease disorder or abnormal physical state or its symptoms in a human being.
In february the edinburgh scotland based company received clearance for the ai enabled device in monitoring patients while in the hospital but this recent.
The restoration correction or modification.
The medical devices and the in vitro diagnostic devices regulations have introduced new responsibilities for the european medicines agency ema and national competent authorities in the assessment of certain categories of medical device.
Some electronic radiation emitting products with medical application and claims also meet the definition of a medical device with examples including diagnostic ultrasound products x ray machines and medical lasers.
The term medical device as defined in the food and drugs act is any article instrument apparatus or contrivance including any component part or accessory thereof manufactured sold or represented for use in.
Understanding the in vitro diagnostic medical devices directive 98 79 ec in vitro diagnostic medical devices ivds are subject to the european directive 98 79 ec ivdd.
First ai medical monitoring wearable approved by fda for home use.
A companion diagnostic device can be in vitro diagnostic device or an imaging tool that provides information that is essential for the safe and effective use of a corresponding therapeutic product.
Medical devices also include in vitro diagnostic products such as general purpose lab equipment reagents and test kits.
The ivdd is implemented in the national laws of the member states.