Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
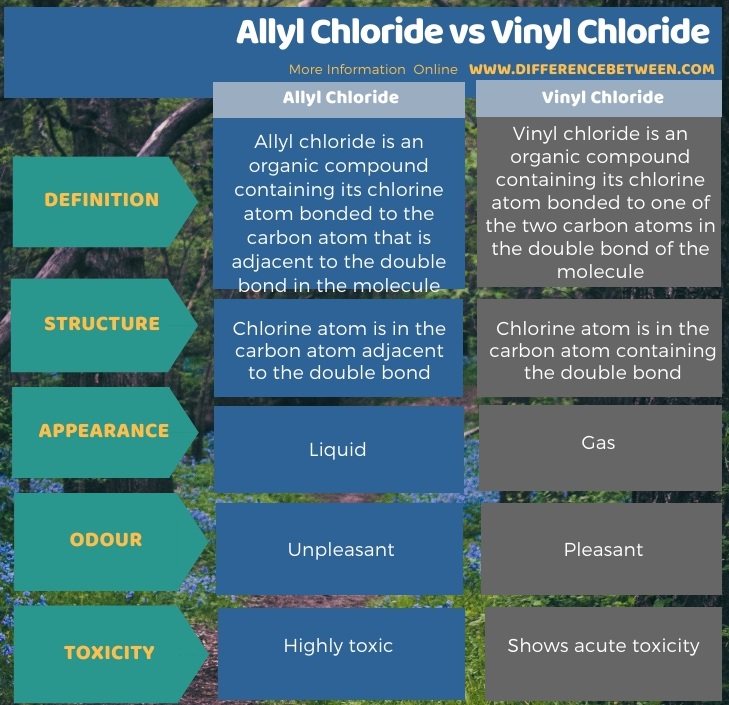
Difference between allyl chloride and vinyl chloride.
The name is derived from the latin word for garlic allium sativum in 1844 theodor wertheim isolated an allyl derivative from garlic oil and named it schwefelallyl.
Allylc compounds are diverse and are used in many industries example.
In easy words we can call allyl group a merger of methylene bridge and vinyl group as ch 2 methylene bridge is attached with ch ch 2 vinyl group and the rest of the molecule to form allyl group.
Allyl chloride has a ch2 group which takes the part in the saturation process and its hydrogens takes part in the hyperconjugation process.
Exposure to allyl chloride primarily occurs for workers in manufacturing plants.
Hydrogen chloride generated in the dehydrochlorination is recycled to the hydrochlorination and dehydrochlorination steps.
Allyl groups have three carbon atoms and five hydrogen atoms.
An allyl group is a substituent with the structural formula h 2 c ch ch 2 r where r is the rest of the molecule.
Ethyl chloride and allyl chloride are organic compounds which have chlorine atoms attached to an organic moiety.
Allyl chloride can be differentiated from vinyl chloride by the silver nitrate test using naoh as allyl chloride gives a precipitate and vinyl chloride does not give any precipitate.
Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom.
The key difference between allyl chloride and vinyl chloride is that ally chloride contains its chlorine atom bonded to the carbon atom that is adjacent to the double bond whereas vinyl chloride contains its chlorine atom bonded to one of the two carbon atoms in the double bond.
The allylic carbon atom is more reactive than normal.
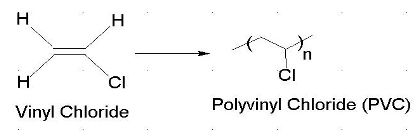
It is mainly converted to epichlorohydrin used in the production of plastics it is a chlorinated derivative of propylene it is an alkylating agent which makes it both useful and hazardous to handle.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
Isomers of allyl chloride are hydrochlorinated to dichloropropanes and dichloropropane is dehydrochlorinated to allyl chloride and its isomers.
Allyl chloride is the organic compound with the formula c h 2 chch 2 cl this colorless liquid is insoluble in water but soluble in common organic solvents.
Propane is chlorinated in the presence of a molten salt to allyl chloride and other chlorinated c3 hydrocarbons.
Allyl chloride is converted into epichlorohydrin and is utilized for the.
Chronic long term exposure to allyl chloride in humans causes injury to the liver and kidneys and the onset of.
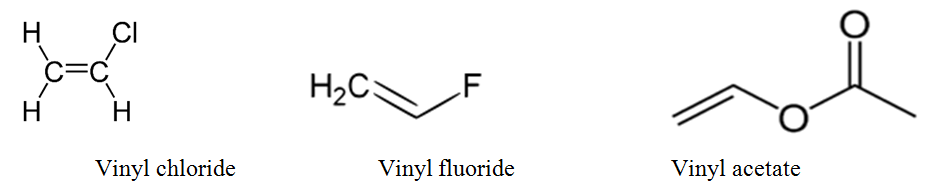
The terms allyl and vinyl are common in organic chemistry because we can use these terms to name compounds using.
The key difference between ethyl chloride and allyl chloride is that ethyl chloride contains a chlorine atom attached to an ethyl group whereas allyl chloride contains a chlorine atom attached to a carbon atom that is adjacent to a double bond.